Pitt’s Center for Vaccine Research Targets Emerging Infections, Biodefense Capabilities
Ted Ross, a professor of microbiology and molecular genetics in Pitt’s School of Medicine, was attending the 2009 Third International Conference on Influenza Vaccines for the World in Cannes, France, when U.S. government officials announced that they had isolated a new strain of flu, H1N1. The new strain comprised both influenza and swine flu, the officials said, and the implication was clear: Influenza had jumped species, moving from pigs to humans.
 Ted Ross’ (center) lab is part of Pitt’s Center for Vaccine Research (CVR), where scientists study a wide range of bacterial and viral pathogens, focusing primarily on emerging infections and biodefense. Ross, a professor of microbiology and molecular genetics in Pitt’s School of Medicine, is an expert in pathogen-host interactions. His research focuses on the development of effective vaccine design for infectious agents such as influenza and HIV. His research team includes research specialists Corey Crevar (left) and Donald Carter (right).
Ted Ross’ (center) lab is part of Pitt’s Center for Vaccine Research (CVR), where scientists study a wide range of bacterial and viral pathogens, focusing primarily on emerging infections and biodefense. Ross, a professor of microbiology and molecular genetics in Pitt’s School of Medicine, is an expert in pathogen-host interactions. His research focuses on the development of effective vaccine design for infectious agents such as influenza and HIV. His research team includes research specialists Corey Crevar (left) and Donald Carter (right).The tone of the Cannes meeting changed drastically, with some participants catching early flights home, and a few prominent international flu researchers scrapping their planned talks in favor of impromptu lectures on zoonoses, or animal viruses that can be transmitted to humans. The Centers for Disease Control and Prevention (CDC) called Ross, an expert in pathogen-host interactions, and asked for help in characterizing the virus in animal models. Ross instructed his team to learn everything they could about H1N1, and they received human samples to test in early June. “There was a lot of anxiety. The World Health Organization (WHO) had raised the alert to pandemic level 6 (widespread human infection), and we were in a race to find answers,” Ross recalled.
Ross’ lab is part of the Pitt’s Center for Vaccine Research (CVR), which comprises 17 full-time faculty and 10 affiliate scientists. The CVR was established the same year as Pitt’s Drug Discovery Institute (2006) and is also housed in Biomedical Science Tower 3. The two entities share the same mission—to improve global health—yet they focus on two different aspects of disease, treatment and prevention. CVR scientists study a wide range of bacterial and viral pathogens, but their main focus is on emerging infections and biodefense. “We don’t mandate what microbes our faculty work on, but they must be epidemic diseases of global importance,” says
Donald S. Burke, associate vice chancellor for global health, dean of Pitt’s Graduate School of Public Health, and CVR director. Burke is a former U.S. Army colonel who led infectious disease research at the Walter Reed Army Institute of Research as well as the Armed Forces Research Institute of Medical Sciences in Bangkok, Thailand.
CVR researchers study pathogens with high levels of antigenic variability. Highly variable viruses (like flu) constantly evolve, creating a challenge for vaccine designers. Ronald Montelaro, a professor of microbiology and molecular genetics and CVR codirector, is a pioneer in the study of lentivruses, the family of retroviruses that includes human immunodeficiency virus (HIV). “We describe lentiviral infection as a virus that enters waving a red flag that is attacked by the immune system. But then the virus throws up a yellow flag or a blue flag, tricking the immune system. A critical question in vaccine development is: How many different colored flags can the immune system effectively recognize and attack?”
Advancing Global Health
While dengue fever is not widely recognized in the United States, it is endemic in more than 100 countries. Caused by a mosquito-borne flavivirus, severe infections can lead to a serious illness called dengue hemorrhagic fever, which is often fatal―particularly in children―and can cause severe complications, such as brain or liver damage, seizure, or shock. CVR researcher Ernesto T.A. Marques Jr., a Pitt professor of infectious diseases and microbiology in the Graduate School of Public Health, travels to Brazil about three times a year to collect data from patients in the South American nation, which is the epicenter of dengue infection. By comparing clinical data with genetic data, he discovered genetic markers that indicate individual susceptibility to dengue infection. Such biomarkers are particularly useful in helping doctors determine which dengue-fever patients may develop serious illness and which may recover quickly.
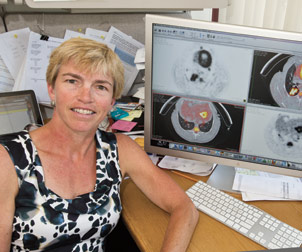 CVR researcher JoAnne Flynn, a professor of microbiology and molecular genetics, and her colleagues have developed imaging technology to better understand how tuberculosis (TB) responds to drugs. The hallmark of TB is the presence of large, inflammatory clumps of bacterial and immune cells, called granulomas. Flynn, using a $12 million grant from the Bill and Melinda Gates Foundation, installed one of the world’s first hybrid positron emission tomography/computed tomography (PET/CT) scanners in a Biosafety Level 3 laboratory, enabling her to watch the granulomas in real time and to see how they respond to different drugs. Flynn’s serial imaging technology represents a major breakthrough in TB imaging and will likely lead to quicker advances in vaccine and drug development.
CVR researcher JoAnne Flynn, a professor of microbiology and molecular genetics, and her colleagues have developed imaging technology to better understand how tuberculosis (TB) responds to drugs. The hallmark of TB is the presence of large, inflammatory clumps of bacterial and immune cells, called granulomas. Flynn, using a $12 million grant from the Bill and Melinda Gates Foundation, installed one of the world’s first hybrid positron emission tomography/computed tomography (PET/CT) scanners in a Biosafety Level 3 laboratory, enabling her to watch the granulomas in real time and to see how they respond to different drugs. Flynn’s serial imaging technology represents a major breakthrough in TB imaging and will likely lead to quicker advances in vaccine and drug development. Tuberculosis (TB) is another devastating disease worldwide, having killed 1.7 million people in 2009. CVR researcher JoAnne Flynn, a professor of microbiology and molecular genetics, and her colleagues have developed imaging technology to better understand how the bacteria respond to drugs. The hallmark of TB is the presence of large, inflammatory clumps of bacterial and immune cells, called granulomas. Flynn, using a $12 million grant from the Bill and Melinda Gates Foundation, installed one of the world’s first hybrid positron emission tomography/computed tomography (PET/CT) scanners in a Biosafety Level 3 laboratory, enabling her to watch the granulomas (what she calls “hot spots”) in real time and to see how they respond to different drugs over the course of a treatment regimen. Previously, researchers were limited to examining tissues postmortem. Flynn’s serial imaging technology represents a major breakthrough in TB imaging and will likely lead to quicker advances in vaccine and drug development.
Flynn’s imaging center is housed in the CVR’s Regional Biocontainment Laboratory (RBL), one of 13 federally funded labs that study potential bioterrorism threats and develop vaccines and therapies for such diseases. RBL Associate Director Kelly Stefano Cole, a professor of immunology in the School of Medicine, notes that in the field of biodefense, vaccine development and drug discovery complement each other. “Of course, we need new vaccines, but if there was a bioterrorist attack and a pathogen was intentionally released, we would need quick access to large stockpiles of therapeutics to treat or blunt the effects of the illness,” Cole says. “Likewise, if a researcher is accidentally exposed to anthrax in the laboratory, we also need drugs to quickly treat that person.”
Burke affirms the commonality between vaccine development and drug discovery. “Both the CVR and DDI are product-oriented centers―the ultimate in translational science. The ideas bubble up from the ground floor of the BST3 and float through the hallways of the various departments, maturing and evolving, until they get to us. Our job is to translate the science into interventions that will improve public and global health.”
Ross believes that the events that transpired during the H1N1 pandemic demonstrated how unprepared our nation is for a major biological emergency. A vaccine was not made widely available to the public until months after the peak of the outbreak. He says, “If this virus had hit the general population as hard as it did young people, the pandemic would have been much more devastating.” He hopes that new-generation vaccines will greatly speed up the flu vaccine production process, lowering manufacturing costs and making vaccines more widely available much more quickly.
So when or where will the next biological emergency occur? No one knows for sure, but these CVR researchers will be on the front lines, ready to fight.
Other Stories From This Issue
On the Freedom Road

Follow a group of Pitt students on the Returning to the Roots of Civil Rights bus tour, a nine-day, 2,300-mile journey crisscrossing five states.
Day 1: The Awakening
Day 2: Deep Impressions
Day 3: Music, Montgomery, and More
Day 4: Looking Back, Looking Forward
Day 5: Learning to Remember
Day 6: The Mountaintop
Day 7: Slavery and Beyond
Day 8: Lessons to Bring Home
Day 9: Final Lessons

